The Chemistry of Water
Properties
Professor Jill Granger
|

IMAGE SOURCE: "Chemistry in Context" Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 "The Wonder of Water"
Water is Weird !?
Ice Floats. That's not weird.... is it?
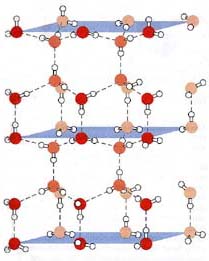
The Structure of Ice IMAGE SOURCE: "Biochemistry", second edition, by D. Voet and J.G. Voet, John Wiley and Sons, Somerset NJ, 1995, Chapter 2 "Aqueous Solutions", pg 31
Water boils at 100°C


IMAGE SOURCE: "Chemistry in Context" Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 "The Wonder of Water" Water is way out of line! It boils at an extremely high temperature for its size. Why? Because of the extensive network of Hydrogen bonds. Those H-bonds are cohesive forces - they want to hold the water molecules together - and there are a lot of them! The process of boiling requires that the molecules come apart: a process that takes a lot more energy than expected.
What's unusual about the freezing point?
How am I affected by these temperature - phase relationships?
Is there anything else?

You've noticed and used water's high heat capacity yourself.
How's that?
|

Selected by the SciLinks program, a service of National Science Teachers Association. Copyright 1999 - 2002 |
|---|
 CONTENTS
CONTENTS  INTRODUCTION
INTRODUCTION  PURPOSE
PURPOSE  SCHEDULE
SCHEDULE  REQUIREMENTS
REQUIREMENTS  PARTICIPANTS
PARTICIPANTS 
H20 - The Mystery, Art, and Science of Water
Chris Witcombe and Sang Hwang
Sweet Briar College
